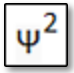A sulfur-free Silicon Thermite
Many a backyard scientist has experimented with thermite reactions, including one of the harder reactions, that of silicon dioxide (silica, SiO2) and aluminium (Al) to produce silicon metal(loid) and alumina. These reactions present great pyrotechnical displays and with a little luck leave you with some lumps of metal as a memento. Because of the metal produced, thermite reactions also find widespread use in aluminothermic metal extraction processes for the preparation of specialist high purity metals and alloys.
Although the reaction 3 SiO2 + 4 Al ---> 3 Si + 2 Al2O3 is thermodynamically favourable, this reaction does not propagate by itself, presumably because the heat of reaction isn't enough to overcome the activation energy it needs. Straight mixes of a silica source and aluminium powder therefore fizzle out or can't be ignited.
To make the reaction self-sustainable, the most used method by backyard scientists (and fellow travellers) is to add a booster mix of aluminium powder and sulfur, which reacts according to 2 Al + 3 S ---> Al2S3 with great development of heat with great development of heat (ΔH ≈ -5.3 kJ/g of stoichiometric mix). This heat provides the missing activation energy and makes the reduction of silica to silicon with Al self-sustaining.
A typical sulfur boosted silicon thermite mixture is silica/Al/S = 100/111/133 (9:10:12) but I've also successfully used mixes much lower in S, such as 100/72/21.
Apart from yielding a self-sustaining reaction, S-boosted silicon thermites have also other advantages:
• Quite easy to ignite, using magnesium (Mg) ribbon (e.g.).
• The resulting slag is a mix of alumina and aluminium sulfide (Al2S3). The much lower MP of the sulfide (around 1,100 C) causes the slag mix to be more fluid than pure alumina, which freezes at around 2,000 C. This greatly helps slag/metal separation, as the slag/metal mix remains liquid longer, allowing it to collect in the bottom of the crucible and the metal to coalesce out. And a mix of alumina and aluminium sulfide is also much softer than pure, fused alumina, making the slag easier to break up mechanically.
• The alumina/aluminium sulphide slag mix reacts readily with water through hydrolysis of the sulphide: Al2S3 + 6 H2O ---> 2 Al(OH)3 + 3 H2S. This breaks up the slag into a (stinky) hydrated alumina slurry (or mud), giving easy access to the metal globules.
You can find an example of a 300 g S-boosted silicon thermite at this blog post of mine.
But that's the good news and there's some bad news too: the aluminium sulfide is so prone to hydrolysis, that even the newly fused slag positively reeks of H2S, in plain English: rotten eggs. Needless to say, adding water or a mineral acid to it, seriously aggravates the problem. Not only does H2S stink terribly, it's also toxic and it's perceptible even in trace amounts.
(Tip: if you're going to treat an alumina/aluminium sulfide slag mix with water, use copious amounts of bleach instead of pure water: the sodium hypochlorite in the bleach will convert much of the H2S to elemental sulfur, which is even recoverable).
In a nutshell, I got so fed up with the smell of rotten eggs, I decided to try and replace the S-booster mix with a sulfur-free system. I chose to investigate a potassium chlorate/Al mix, which reacts according to 2 Al + KClO3 ---> Al2O3 + KCl with an estimated ΔH ≈ 7.11 kJ/g (of stoichiometric mix) heat generated. I had used such mixes before for lighting thermites.
Initial tests with a silica/Al/KClO3 = 100/72/27 mix showed clearly that the reaction proceeded self-sustainingly and that Si metal was formed, in a hard, porous alumina matrix. I gradually stepped up the amount of booster mix to 100/84/57 and later to SiO2/Al/KClO3 = 100/96/81, to find that progressively more of the slag ends up at the bottom of the crucible (I used mostly 20 g mini batches for the development work) because of the increasingly high peak temperatures during the reactions.
Much of this development can be followed here and on subsequent pages at the ABYMC forum (where I post as Gert from England).
The main problem remains slag/metal separation, both in situ and after the reaction products have cooled down: the pure, fused alumina freezes up quickly into a very, very hard mass. 32 w% HCl doesn't even begin to dent it and forget about mechanical separation: this stuff is HARD!
I then proceeded to test calcium fluoride (Fluorite, CaF2, calcium fluoride) as a potential flux, at 20 w% added to the promising 100/96/81 formulation (this then became 100/96/81/55 - with 55 the CaF2) . Although it made a world of difference in the sense that much larger globules of Si metal form, the slag remains extremely hard and insensitive to HCl. Another test at 40 w% CaF2 showed that at that level the reaction was being slowed down, probably due to adding so much inert material, and hence slag metal separation deteriorated again due to lower peak temperatures.
The purpose of the CaF2 is essentially that of slag fluidiser. Fluorite is inert in these conditions and takes no part in any chemical reactions but has a much lower melting point than pure alumina: 1402 C (2555 F) for fluorite against 2054 C (3729 F) for alumina. Adding relatively small amounts of the lower melting fluorite to the thermite mix therefore helps keeping the slag as fluid as possible for as long as possible, thereby allowing the slag metal mix to collect at the bottom of the crucible and the metal and slag to separate out by gravity.
So far the best formulation has been found to be SiO2 / Al / KClO3 / CaF2 = 100 / 96 / 81 / 55 (all parts by weight), which has been tested in a 100 g thermite mix with good results, although there is room for improvement. Slag/metal separation is generally good with large blobs of pure silicon metal being formed but adhesion of the slag to the metal remains a problem. Further attempts at optimising will include increasing the portion of booster mix and flux to obtain a mix that runs even hotter and should by rights lead to even better slag/metal separation. Another possibility is to use a flux that is even lower melting, such as Cryolite (Na3AlF6, sodium hexafluoroaluminate). Expect updates!
The principle of chlorate-boosted thermite reactions has in the mean time also been successfully deployed on an even more notoriously difficult reaction, that of extracting titanium metal from titanium dioxide. And the production of ferrotitanium (60 w% iron - 40 w% titanium) without any booster has also been demonstrated separately.
A simple but effective thermodynamical model of what goes on thermally inside a thermite mix has also been developed and has been shown to be accurate in estimating the peak temperatures during reaction. More on that in a dedicated post to come soon.
Here's an example of a copper thermite reaction.
Related reading: Manganese thermite from manganese (II) oxide.