Copper thermite!
In a previous post I described how to carry out a silicon thermite reaction to obtain small amounts of silicon metal. In this post I'll describe a copper thermite reaction, based on the same principles. Those uninitiated in thermite reactions are encouraged to read the silicon thermite post first to get a good idea of the basics.
In a copper thermite reaction, copper oxide is reacted with aluminium powder to yield pure copper metal and aluminium oxide (or alumina), accompanied by a great deal of heat. The word equation is therefore:
copper oxide + aluminium ---> copper metal + aluminium oxide (+ heat of reaction)
In the case of copper, this metal and chemical element yields two distinct types of oxides, one known as copper (I) oxide, the other as copper (II) oxide. The former is also known as cuprous oxide, the later as cupric oxide. Their chemical formulas are respectively Cu2O and CuO.
Copper thermites are notoriously exothermic, i.e. they produce lots of reaction heat. Of both oxides, the copper (I) oxide is the one that generates the least heat and that's why I chose to run it today.
In chemical terms the balanced reaction equation is:
3 Cu2O + 2 Al ---> 3 Cu + Al2O3 + ΔH
(with ΔH [delta H] the reaction heat).
The reaction equation allows to calculate the stoichiometric ratio between the amount of copper (I) oxide and aluminium powder. This ratio Cu2O:Al is approximately 8:1 (by weight).
After lighting the magnesium fuse, I removed myself to a safe distance. The mixture ignited swiftly and burned right through with bright light and considerable sparks, in a matter of 5- 10 seconds flat. Too short a time to take any pictures but you can see a video and pictures of a similar copper (I) thermite reaction here (scroll down a little).
Here are parts of the egg cup (shattered due to thermal stress) after soaking in brick cleaner. Brick cleaner or patio cleaner contain hydrochloric acid which removes the copper oxidation and also dissolves the aluminium oxide, leaving the pure copper metal behind. The blob of copper in the centre of the picture is the same one pointed to in the paragraph above. A clean 1 penny coin is included for comparative purposes.
Related post: Making homemade bronze, bronze age-style.
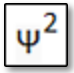
7 Comments:
Being a hunter, usually in very cold weather, many of us use "Hand Warmers". I believe they contain these same two metals (seperated until activated). They work for about 8 hours. I use both types, one for the feet and the other for inside your gloves...
Cookie:
I'm gonna have to dissapoint you there. Thermites either burn so hot they can practically melt titanium or they simply don't do anything at all. There is really no slow or "controlled" thermite reaction: it would burn your hands off; iron based thermites are used in incendiary bombs!
Undoubtedly yur right...chemistry has never been one of my strong suits. I do know that they use some sort of metals that when the reaction takes place it produces a goodly amount of heat...I love those things...especially on sub zero days....
charcoal, iron powder, water and zeolite, or a zeolite substitute are the basic ingrediants in any of the hand warmers I've looked at.
Hey, Im a chemistry student in the united states and I'm looking to do a copper thermite reaction...but I need a legit. reference to do this....is there any way I could get your references?!?!?! it would be completely and amazingly helpful!!!!!!!!!!!!
Reaction isn't balanced.
As written, there are six copper atoms on the left (3 Cu2O) and only three copper atoms on the right (3 Cu). Should be 6 Cu on the right.
Handwarmers usually use iron/water (producing iron oxide and heat) but supersaturated sodium acetate (which is exothermic as it crystallizes) can also be used.
Sodium acetate is also used in hot ice. See here-http://www.youtube.com/watch?v=aC-KOYQsIvU
Post a Comment
<< Home